One strategy by the N2Africa project to enhance legume productivity in Africa was to select rhizobial strains with enhanced biological nitrogen fixation (BNF) efficiency from the various countries (as part of the GL x GR x E x M framework) for use as inoculants. In line with this goal, I isolated rhizobia from beans growing in several agro-ecologies in Kenya and first assessed their genetic diversity. Diversity studies not only identify rhizobial strains but also inform on the predominance of strains, their movement, and on the dynamics of genetic material exchange. Only a few studies had previously genetically characterized rhizobia that nodulate beans in Kenya (e.g. Anyango et al. Appl. Env. Microbiol. 61:4016-4021, 1995) and their limited scope strengthened the justification for a diversity assessment. My investigations revealed a considerable diversity at the strain level, while at the species level, at least five species of Rhizobium were found to nodulate beans in Kenya.
In addition to genetic diversity, I assessed the BNF efficiency of the strains. The strain commonly used to inoculate bean in Kenya, R. tropici CIAT 899, was isolated from Colombia and there is evidence that its mixed success in Kenya is sometimes due to poor adaptation to certain edaphic conditions. A time-tested approach for the discovery of adapted inoculants involves the selection of strains with enhanced BNF efficiency from the areas targeted for inoculation, or from climatically matched environments. To identify potential inoculant strains for the Kenyan bean inoculant industry, I assessed the effectiveness of Kenyan strains on Kenyan bean cultivars in controlled glasshouse conditions. Eleven of the strains tested had BNF efficiencies equal to that of CIAT 899 but will need to undergo field testing to ascertain their elite status further.
I also carried out rhizobia competition studies. Soils used to grow beans in Kenya, and indeed most parts of Africa, have high densities of indigenous rhizobia (e.g. Amijee & Giller. Afr. Crop Sci. J. 6(2):159-169, 1998) that hinder responses to inoculation. Therefore, unless the issue of competition is addressed, achieving inoculation response in beans is likely to continue being problematic. One solution to the competition problem is to screen rhizobia for competitiveness and use the most competitive as inoculants. Hence, I used marker genes, which enable nodule occupancy to be visualized after a staining step, to evaluate the competitiveness of >40 Kenyan strains against a reference, CIAT 899. Results indicated mixed competitiveness against CIAT 899, with both highly competitive and highly uncompetitive strains identified. These results present at least two opportunities. The first and more obvious opportunity is for the use of the competitive strains as inoculants. The second is for targeting of areas in which to inoculate beans. This latter prospect was further supported by my observations that seed-applied CIAT 899 occupied most of the nodules in soils containing up to 106 cells (g-1 of soil) of the highly uncompetitive strains. Signifying, in areas where the highly uncompetitive rhizobial genotypes are prevalent, responses to inoculation are likely to be achieved. Other interventions such as increasing inoculation dosage did not enhance the inoculant’s nodule occupancy in soils with high rhizobial densities. For more on my research, see my thesis: http://researchrepository.murdoch.edu.au/id/eprint/36067/
 |
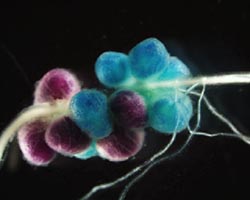
Left: Dr George Mwenda (second from right) with PhD supervisors (all from the Centre for Rhizobium Studies at Murdoch University, Perth, Australia). Supervisors from left to right are Dr Jason Terpolilli, Prof. John Howieson, and Dr Graham O’Hara Right: Double stained nodules after inoculation with CIAT 899-gusA (blue) and NAK 104-pGM1 (magenta). Photo was also published in Podcaster 32 |
 |
Lastly, let me take this opportunity to thank N2Africa for funding my PhD (in partnership with Murdoch University) and my supervisors (see photo) for their great academic support. My PhD degree was conferred recently. I look forward to my continued contribution to the BNF research.
George Mwenda, Centre for Rhizobium Studies, Murdoch University, Perth, Australia
