Introduction
| Striga parasitism can devastate maize fields in Kenya The parasitic weed striga (Striga hermonthica) is able to draw nutrients directly from a suitable host plant such as maize through a haustorium, an underground root-root connection. In regions of sub-Saharan Africa (SSA) including Western Kenya, striga can cause up to 70-100% yield loss in fields of maize and other crops (Khan et al., 2006) (Fig. 1). |

|
| Strigolactone exudates allow striga to germinate and parasitize maize The strigolactones (SLs, Fig. 2) are a group of plant hormones involved in regulating beneficial symbioses, for example between mycorrhizal fungi and maize. However, maize-exuded SLs can also induce striga to germinate, locate, and infect roots of the host plant. |
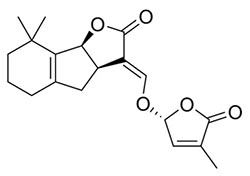
|
| In Kenya, legume species are often rotated or intercropped with maize, which can increase yields due to nitrogen fixation, as well as provide food security (Fig. 3). Legumes also produce and exude SLs, associated with establishing effective nitrogen fixing symbioses with rhizobial bacteria. Striga is an obligate parasite, but unable to form a connection with most legumes, resulting in germination and death of the weed upon detection of legume-derived SLs. This non-nitrogen benefit of legumes can reduce the striga seed bank in a field, and reduce parasitism in a subsequent maize crop by up to 70% (Khan et al., 2006). However, the large environmental diversity present in smallholder systems has made characterization of this effect a challenge, and warrants further study. | 
|
Hypothesis
It is hypothesized that rotating maize with legumes will reduce the striga seed bank and subsequent parasitism of a maize crop in a variety of environments.
Objectives
1. Quantify striga seed bank in maize fields of high and low infestation.
2. Correlate seed bank and striga emergence with soil physiochemical characteristics.
3. Compare striga emergence and seed bank between maize in monoculture or rotation with a legume crop.
Methodology
|
Selection of field sites Soil and seed bank analysis |
|
Field experimentation
In each field two treatments of either legume or monoculture maize will be planted, with high/zero N fertilization treatments to disentangle the legume N2-fixation benefit. Striga reduction in the subsequent maize crop will be compared between the cropping treatments with visual inspection, and analysis of the seed bank as described above.
Laboratory experimentation
In a side-project, several barley mutants have been generated by a collaborator which will be used as a model system to examine altered SL exudation. In these plants, three key genes involved in the biosynthesis of SLs via the common symbiosis pathway have been repressed. The plants will be grown alongside striga seed in lab and greenhouse experiments, and the induced striga germination will be compared to a barley wildtype control. Later, genotypes of interest will be analysed for unique strigolactone exudation profiles compared to the wildtype. It is anticipated that this portion of research will provide mechanistic understanding of how components of the common symbiosis pathway contribute to SL production and striga parasitism.
Conclusions
This research will provide updated understanding of the degree by which striga parasitism can be combated by legumes, offering promise to control striga in Western Kenya. Future work will include further characterization of the striga-reducing benefit provided by legumes.
Acknowledgements
In addition to N2Africa, Travis’ research is supported by a grant from the Natural Sciences and Engineering Council of Canada.
Travis Goron, Wageningen University & Research, The Netherlands
References
Gacheru E, Rao MR. 2001. Managing Striga infestation on maize using organic and inorganic nutrient sources in western Kenya. Int J Pest Manage 47: 233-239.
Khan ZR, Midega CAO, Hassanali A, Pickett JA, Wadhams LJ. 2006. Assessment of different legumes for the control of Striga hermonthica in maize and sorghum. Crop Sci 47:730-734.
Yoneyama K, Xie X, Sekimoto H, Takeuchi Y, Ogasawara S, Akiyama K, et al. 2008. Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol 179:484–492.
